Poly(ethene) is produced in three main forms:
low density (LDPE) (< 0.930 g cm-3)
linear low density ( LLDPE) (ca 0.915-0.940 g cm-3)
and high density (HDPE) (ca 0.940-0.965 g cm-3).
The process is operated under very high pressure (1000-3000 atm) at moderate temperatures (420-570 K)
This is a radical polymerization process and an initiator, such as a small amount of oxygen, and/or an organic peroxide is used.
Ethene (purity in excess of 99.9%) is compressed and passed into a reactor together with the initiator. The molten poly(ethene) is removed, extruded and cut into granules. Unreacted ethene is recycled. The average polymer molecule contains 4000-40 000 carbon atoms, with many short branches.
HDPE is produced by three types of process. All operate at relatively low pressures (10-80 atm) in the presence of a Ziegler-Natta or inorganic catalyst. Typical temperatures range between 350-420 K. In all three processes hydrogen is mixed with the ethene to control the chain length of the polymer.
The Ziegler-Natta catalyst, as granules, is mixed with a liquid hydrocarbon (for example, 2-methylpropane (isobutane) or hexane), which simply acts as a diluent. A mixture of hydrogen and ethene is passed under pressure into the slurry and ethene is polymerized to HDPE. The reaction takes place in a large loop reactor with the mixture constantly stirred (Figure 4). On opening a valve, the product is released and the solvent is evaporated to leave the polymer, still containing the catalyst. Water vapour, on flowing with nitrogen through the polymer, reacts with the catalytic sites, destroying their activity. The residue of the catalyst, titanium(IV) and aluminium oxides, remains mixed, in minute amounts, in the polymer.
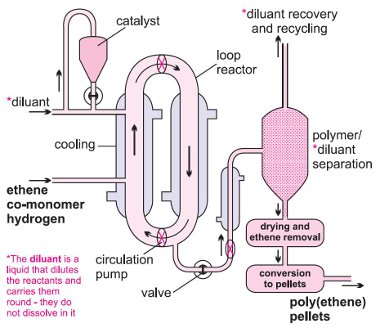
The second method involves passing ethene and hydrogen under pressure into a solution of the Ziegler-Natta catalyst in a hydrocarbon (a C10 or C12 alkane). The polymer is obtained in a similar way to the slurry method.
Ethene polymerizes to form grains of HDPE, suspended in the flowing gas, which pass out of the reactor when the valve is released.
Modern plants sometimes use two or more of the individual reactors in series (for example two or more slurry reactors or two gas phase reactors) each of which are under slightly different conditions, so that the properties of different products from the reactors are present in the resulting polymer mixture, leading to a broad or bimodal molecular mass distribution. This provides improved mechanical properties such as stiffness and toughness.
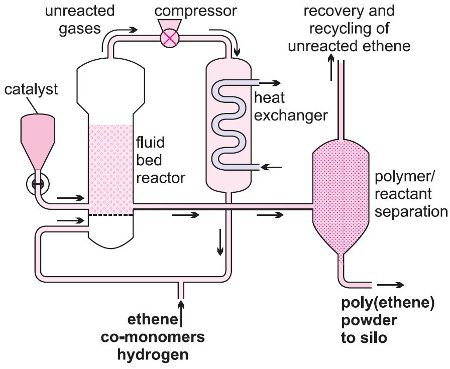
Low density poly(ethene) has many uses but the high pressure method of manufacture by which it is produced has high capital costs. However, an elegant technique has been developed, based on both Ziegler-Natta and inorganic catalysts to produce linear low density poly(ethene) LLDPE, which has even improved properties over LDPE. Any of the three processes, slurry, solution and gas phase, can be used when a Ziegler-Natta catalyst is chosen. The gas phase process is used when the inorganic catalyst is employed.
The structure is essentially linear but because of the short chain branching it has a low density. The structure gives the material much better resilience, tear strength and flexibility without the use of plasticisers. This makes linear low density poly(ethene) an ideal material for the manufacture of film products, such as those used in wrappings.
The properties of the polymer, and hence its uses, can be varied by varying the proportion of ethene and co-monomer and by using different co-monomers. All this can be done without shutting down the plant, an enormous advantage.
The LDPE or LLDPE form is preferred for film packaging and for electrical insulation.
HDPE is blow-moulded to make containers for household chemicals such as washing-up liquids and drums for industrial packaging. It is also extruded as piping.
Many plants can produce both forms of poly(ethene) and alter the amount that they produce of each type at short notice. Both use a Ziegler (or Phillips) catalyst. If pure ethene is used, HDPE is formed. LLDPE is produced when a small amount of another alkene, for example but-1-ene, is added to the ethene. Another form, mLLDPE, is, at present, produced in much smaller quantities.
Sources: http://www.essentialchemicalindustry.org/poly
For LLDPE, HDPE process, whether slurry or gas phase applied, it needs pentane or hexane for the catalyst carriage. Dongying Junyuan's pentane and hexane , have been exported to SABIC, FORMASA PLASTICS, SINOPEC etc for the polymerization since 2009, through its branch Dongying Liangxin Petrochemical. Contact us for more infomation now!
附LLDPE工艺
在气相LLDPE工艺问世之前,聚乙烯的生产限于采用高压(釜式或管式法)和较低压力的溶剂和淤浆法工艺。这些工艺以其特定的产品市场为目标,分别生产HP-LDPE、MDPE和HDPE。每一种工艺仅能生产有限密度变化范围的产品。气相LLDPE工艺问世后,使此情况发生很大变化。它可用同一反应器生产所有密度范围的PE产品,能灵活地根据市场需求变化,改变所生产的PE品种。
现在,LLDPE树脂可用液相和气相工艺进行生产。液相工艺中,Dow Chemical的冷却低压法和NOVA 化学公司的中压法占压倒优势。这两种工艺均可切换生产LLDPE和HDPE。虽然历史上淤浆法以生产HDPE和MDPE为主,但现在已可生产LLDPE和塑性体。此外,LLDPE也可用高压釜和管式反应器制造。
UnivationTechnologies和BP公司控制了气相法LLDPE生产技术的转让。气相法技术也能切换生产LLDPE和HDPE。但如前所述,由于产品牌号切换会产生大量不合格的过渡产品,经济上不合算。因此,通常的做法是,一套装置在一段时间内专用于生产一种主要产品,而在另一段时间内生产另一种产品,不经常进行产品切换。Univation的低压气相流化床工艺,亦即UnipolTM工艺是生产LLDPE的最普通工业化工艺。在此工艺中,乙烯和共聚单体(1-丁烯或1-己烯)在流化床反应器中聚合,生成颗粒状聚合物。其特点是将一种载体型钛或钛-铬催化剂粉末连续送入流化床反应器(fluid bed reactor),并连续地由反应器取出聚合物产品颗粒。在流化床中,增长的聚合物颗粒被循环的乙烯/共聚单体物流流态化。循环物流通过外部冷却器冷却,除去反应热。反应器压力约为300磅/平方英寸,反应温度约为88℃。UnipolTM工艺也可用于生产聚丙烯,采用Shell的超高活性催化剂(SHAC)。BP的低压气相流化床工艺与UnipolTM工艺非常相似。仅冷凝液送入流化床的方式稍有不同。BP的方法是先将冷凝液与循环物流分离,
然后用置于流化床内的喷咀雾化,将其送入流态化床层。UnipolTM则不进行分离,冷凝液随循环物流一起送入流化床反应器。
Montell(现Basell)的Spherilene工艺能生产密度范围为0.890-0.970克/立方厘米的可裁缝分子量分布的聚乙烯。此工艺采用一台小的液相环形反应器与一或二台气相流化床反应器串联,可使用C2-C8烯烃共聚单体的混合物,在反应器内生成掺混物和合金。因此工艺可直接得到球形聚合物,故可取消挤出造粒工序。
Dow生产线性聚乙烯的低压溶剂法工艺已用于世界上许多工厂,但这些工厂均属Dow的自有工厂。在此工艺中,乙烯、1-辛烯和C8-C9异构链烷烃溶剂与改性的Ziegler催化剂溶液一起送入两台串联的搅拌反应器。反应在395磅/平方英寸和160℃的条件下进行。第二台反应器溶液中,聚合物的含量为10%。总停留时间为30分钟。反应器的流出物在35磅/平方英寸的绝压下闪蒸,除去溶液中的乙烯。继之,用加热/闪蒸步骤除去溶剂。聚合物则进行挤压造粒。
生产线性聚乙烯的中压SclajrTM溶液法工艺系由DuPon Canada开发,已转让给世界上20多家公司。在1994年中期,NOVA Chemicals购买了Sclai尸技术及其世界技术转让业务,并采用新一代的非茂金属催化剂,提出了SclairIITM技术。
Phillips的淤浆环管反应器工艺主要用于生产HDPE,但1993年Phillips借采用新的铬氧化物催化剂,成功地降低了聚乙烯的密度范围,得到两种低密度(0.923和0.927克/立方厘米)线性聚乙烯。1995年,又报导采用茂金属催化剂可将密度进一步降低至0.910克/立方厘米。
Sources:http://3y.uu456.com/bp_8il9s4ed0k4oweg0pire_1.html


